P. Prabhu
Dr. P. Prabhu is Assistant Professor (Sr. Gr) in the Department of Physical Chemistry, University of Madras.
Awards
Professors can receive various awards to recognize their teaching, research, service, and contributions.
Academic
To assess learning, critical thinking, creativity, and application of knowledge in a particular field of study.
Dr. P. Prabhu, M.Sc., Ph.D
Assistant Professor, Department of Physical Chemistry, University of Madras, Chennai.
Our research interest include synthesis of noble metal(Au, Ag, Cu) nanoclusters, less known transition metal (Fe, Co, Ni) nanoclusters, Carbon Quantum Dots (CQDs), Lead free Perovskite materials for biomedical, sensing, catalytic and light harvesting applications.
RESEARCH INTEREST
Metal nanoclusters are ultrasmall nanoparticles with size ranging from several to hundred of metal atoms. They posses higher stability. They also exhibit unique properties compared to their bulk counterparts due to their strong quantum confinement effect. This class of nanomaterials provides a new platform for diverse applications such as catalysis, bioimaging , sensor, energy harvesting etc.
Carbon quantum dots (CQDs) are typically quasi-spherical nanoparticles comprising amorphous to nanocrystalline cores. The presence of carboxyl moieties on the CQD surface imparts excellent water solubility and suitable chemically reactive groups for further surafce passivation, hence the fluorescence properties are enhanced. The extensive photochemical stability of CQDs render them very attractive for technical applications.
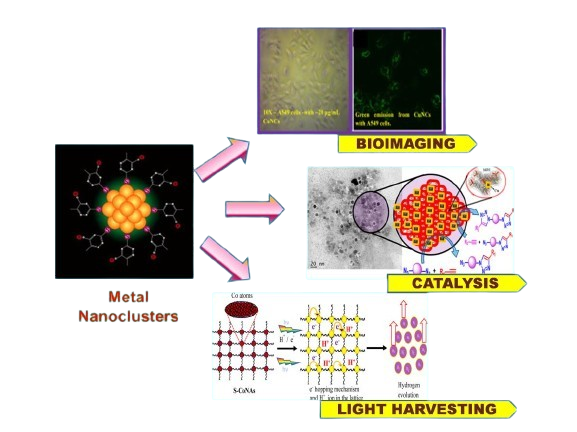
Atomically precise metal nanoclusters exhibit unique electrochemical properties that are very sensitive to the change in their size, shape, and composition. Electrochemical methods are very informative about their electronic structures especially near the HOMO and LUMO levels. Metal nanoclusters with a well-defined core−shell structure may be of special advantage because the core and shell can separately be engineered to show suitable binding properties for electrocatalysis. Their redox potentials can additionally be tuned by their size, shape and composition for them to act as an efficient electron transfer mediator for electrocatalysis as well as for sensing.

